Rapidly reverse dilation and redefine your patient experience.
Starts working in 30mins.
Rapidly reverses dilation, generally by 90mins!
Don't let eye dilation disrupt the rest of their day.
What you need to know
Indication
RYZUMVI (phentolamine ophthalmic solution) 0.75% reverse mydriatic is indicated for the treatment of pharmacologically-induced mydriasis produced by adrenergic agonists (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents.

How Ryzumvi works
Ready, Set, Ryzumvi!
- Ready
Ensure Ryzumvi is always on hand. Make it one of your eye dilation tool-kit essentials. - Set
Store Ryzumvi refrigerated at 2°C to 8°C (36°F to 46°F). - Ryzumvi
Keep a foil pouch handy in your exam areas, ready to offer patients who choose to get on with their day.
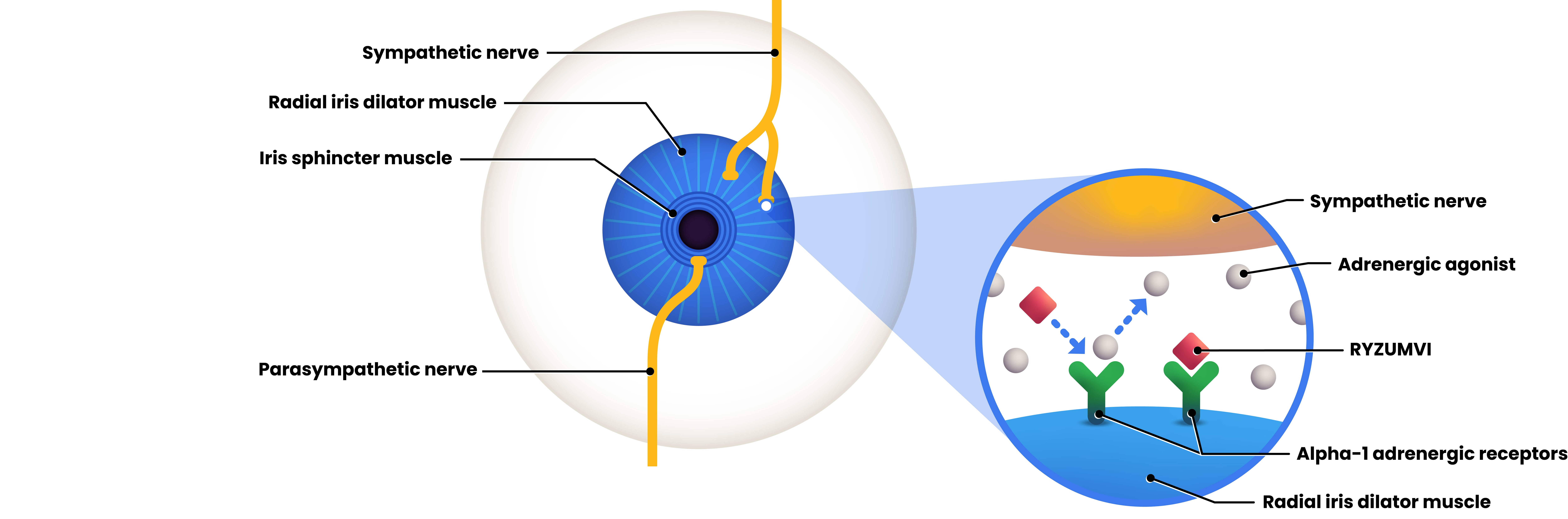
1. The dilation pathway
RYZUMVI reversibly binds to alpha-1 adrenergic receptors on the radial iris dilator muscle, reducing pupil diameter and directly antagonizes the mydriatic effect of an alpha-1 adrenergic agonist.
2. The constriction pathway
RYZUMVI indirectly reverses dilation induced by muscarinic antagonist effects on the iris sphincter muscle, regardless of whether phenylephrine, tropicamide, or Paremyd® is used.
Select important safety information
Warnings and precautions:
- Uveitis: RYZUMVI is not recommended to be used in patients with active ocular inflammation (e.g., iritis).
- Potential for eye injury or contamination: Care should be taken to avoid touching the vial tip to the eye or to any other surface.
- Use with contact lenses: Contact lens wearers should be advised to remove their lenses prior to the instillation of RYZUMVI and wait 10 minutes after dosing before reinserting their contact lenses.
- Adverse Reactions: The most common adverse reactions that have been reported are instillation site discomfort (16%), conjunctival hyperemia (12%), and dysgeusia (6%).